
Mechanism:
Physiological an Oncogenic Acdtivation of RET Kinase
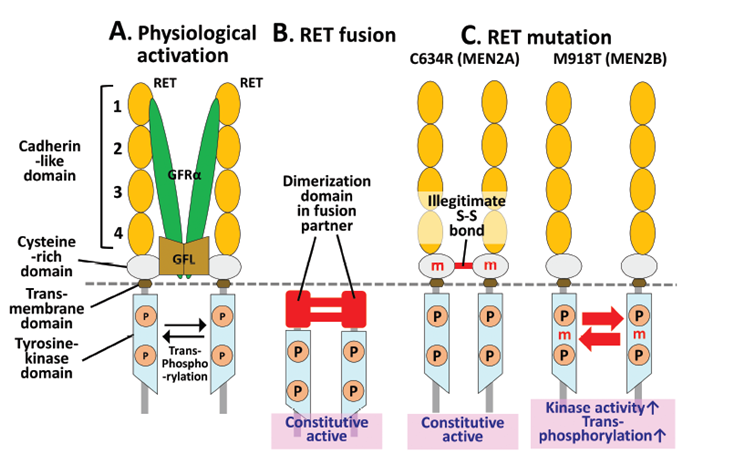
Oncogenic activation of RET occurs via two genetic mechanisms:
somatic RET fusion, somatic and germline RET mutations
2020. Carcinogenesis
Resistance Mechanism of Selective RET Inhibitors
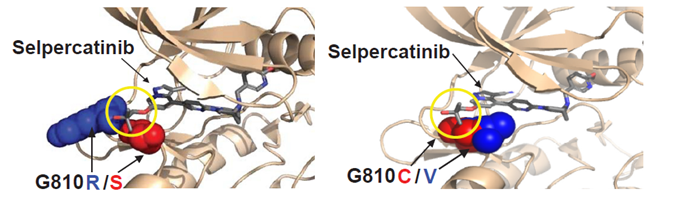
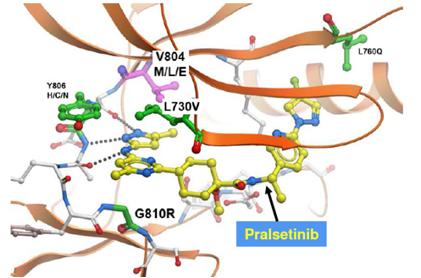
RET solvent front mutation RET G810R/S/C/V block
the binding of first generation RET inhibitors
2020 . J Thorac Oncol ; 2020. NACLC
Indications:
APS03118 has therapeutic potential for RET altered cancers not limited by tumor type. RET fusion and rearrangement occur in approximately 1-2% of NSCLC patients , particularly in young, non-smoking lung adenocarcinoma patients with an incidence of up to 7-17%. RET mutations are detected in 90% of medullary thyroid cancers and RET fusion in 10-20% of thyroid cancer. In addition, less than 1% of other solid tumors such as pleural mesothelioma, colorectal cancer, esophageal cancer, breast cancer, pancreatic cancer, and pancreatic cancer are driven by RET aberration. About 70,000 to 80,000 patients worldwide with advanced solid tumors can benefit from monotherapy with RET inhibitors according to 2019 global data. RET inhibitors also have shown synergy with other targeted therapies, such as RET mutations after resistance to EGFR inhibitors, and becomes the optimal choice for unresponsive and refractory RET positive patients.

Address
Office: San Diego,US
Office: Building 10-1, No.2 Jingyuan North Street, BDA, Beijing, China
Tel
+86-10-67860673
