
News
Focus on the latest innovative drug technology, deliver the therapeutic hopes for patients

Image source: CDE official website
RET protein is a membrane receptor tyrosine kinase. RET gene fusion and mutation can lead to over-activation of RET signaling pathway and thus participate in the tumor development. The aberrant activation of RET mutation are key drivers of the genesis of various tumors.
Currently, there are only a few drugs targeting RET, and only the first-generation RET inhibitors Selpercatinib and Pralsetinib have been granted the accelerated approval. Both of these drugs have finally developed drug resistance and caused disease progression in patients. As no drug could address RET resistance mutation, there is a huge unmet clinical need.
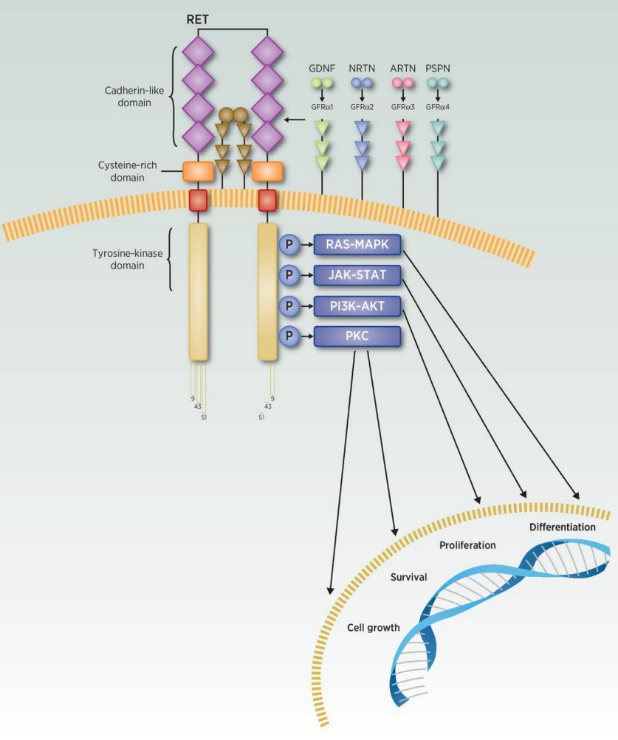
Image Source:Clin Cancer Res 2020;26:6102-11
APS03118 is a small molecule drug with completely independent intellectual property rights. It is a novel second-generation RET inhibitor with high potency and selectivity. APS03118 inhibits tumor cell proliferation, migration and differentiation by selectively inhibiting RET protein and blocking downstream signaling pathways (including RAS, MAPK, ERK, PI3K, AKT, etc.).
Preclinical data have shown that APS03118 has a clear mechanism of action and consistent biological activities in vitro and in vivo. It is reported that the resistance mechanisms of first-generation RET inhibitors are mainly the RET G810R/S/C solvent front mutations(SFMs), also the L730I/M roof, RET V804M/L/E gate keeper and RET Y806H hinge mutations. APS03118 can potently inhibit the above drug resistance mutations, and induced up to 90% tumor growth inhibition in the SFM G810R mouse xenograft and was well tolerated.
APS03118 showed little inhibition of other kinases in 468 kinases selectivity experiments, and was highly selective for RET, thus may have low toxicity and high safety characteristics. APS03118 has potent tumor suppressive activity in mouse brain tumor model, the tumor of APS03118 treatment group completely subsided, and the survival rate of mice was 100%, indicating that this product may have therapeutic advantages for patients with brain metastases, which also suggests that it has a broad clinical application prospect.
Dr. Jun Zhong, vice president of R&D of APS, stated: “We are very pleased that the clinical trial of APS03118 has been approved in China. This product has a stronger inhibitory effect on multiple RET aberrances, not only can effectively overcome the resistance caused by the first-generation selective RET inhibitors, but also shows great potential in first-line treatment. The approval can promote the latest domestic cancer therapies to keep up with the international frontier, and bring new hope to more cancer patients. We will make every effort to promote clinical trial and look forward to the early approval of APS03118 for the benefit of the majority patients.”
Applied Pharmaceutical Science, Inc. is a biopharmaceutical high-tech company focused on innovative cancer precision therapy and tumor-driven gene drug development through in-depth exploration of cancer-causing driver genes, protein structure and function, and synthetic medicinal chemistry. APS aims to explore the life sciences, innovate precision cancer therapy, and seek new hope for cancer patients worldwide with breakthrough tumor precision therapies.
Please contact us for further communication about company and products:
info@apspharm.com

Address
Office: San Diego,US
Office: Building 10-1, No.2 Jingyuan North Street, BDA, Beijing, China
Tel
+86-10-67860673
